Case Studies
RAPSKEY by Smart Pill Solution
The digital transformation of regulatory affairs in Algeria: towards smoother and more efficient management.

Problématique
Challenge and objectives
Observations
50%
18%
60%
67%
Strategy
The digital transformation of regulatory affairs in Algeria: towards smoother and more efficient management.
Wireframes

Interfaces
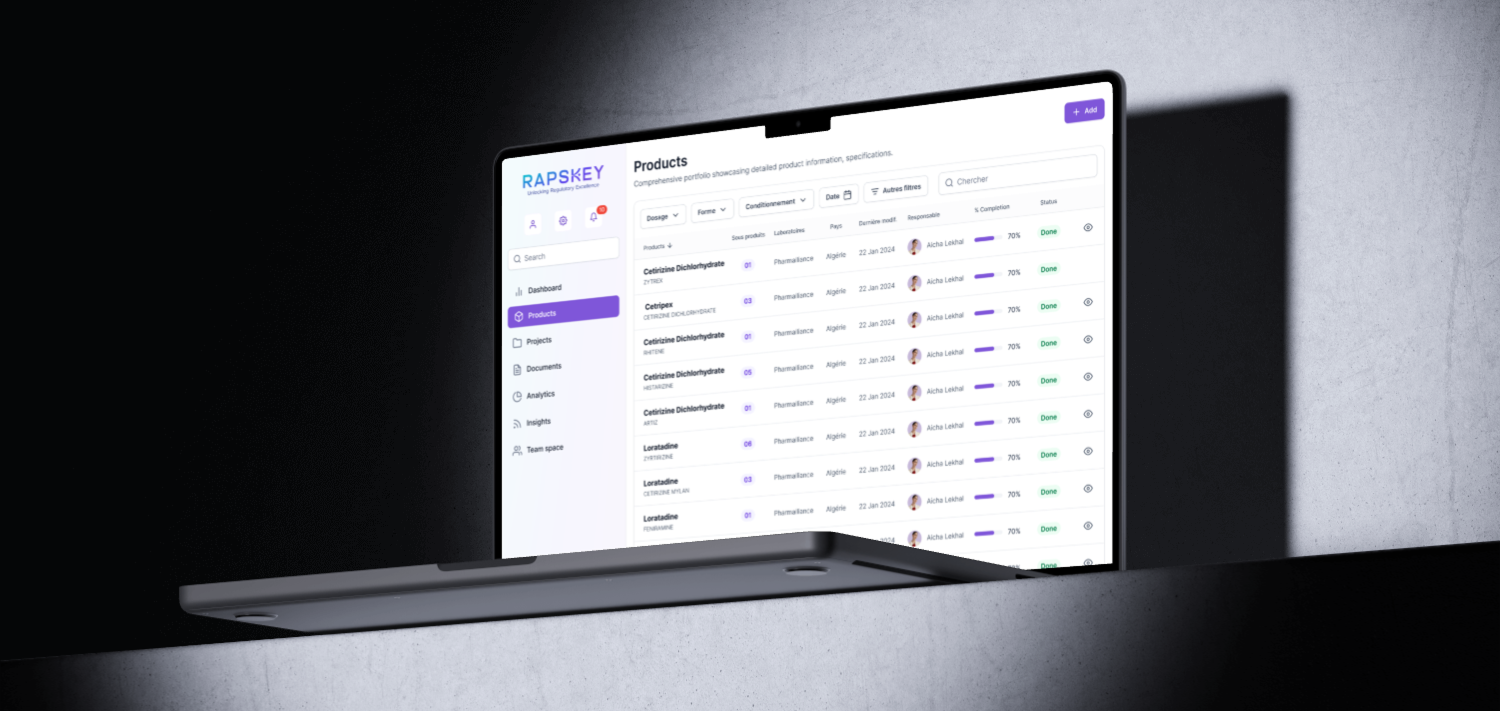
Testimonial
Results
After several development sprints, Rapskey has become a powerful tool that:
- Significantly reduces the time needed to process regulatory files.
- Reduces human error, improving compliance with health authorities.
- Centralizes all regulatory information in a single platform.
- Automates deadline reminders, preventing registration delays.
Final result
By partnering with Qantra, the company behind Rapskey now has an innovative solution that revolutionizes regulatory affairs management.
Have a Specific Need?
We adapt our solutions to offer you the best of digital technology.





